shelf life calculator medical device
Establishing Shelf Life of Medical Devices. Accelerated Aging Time Calculator.
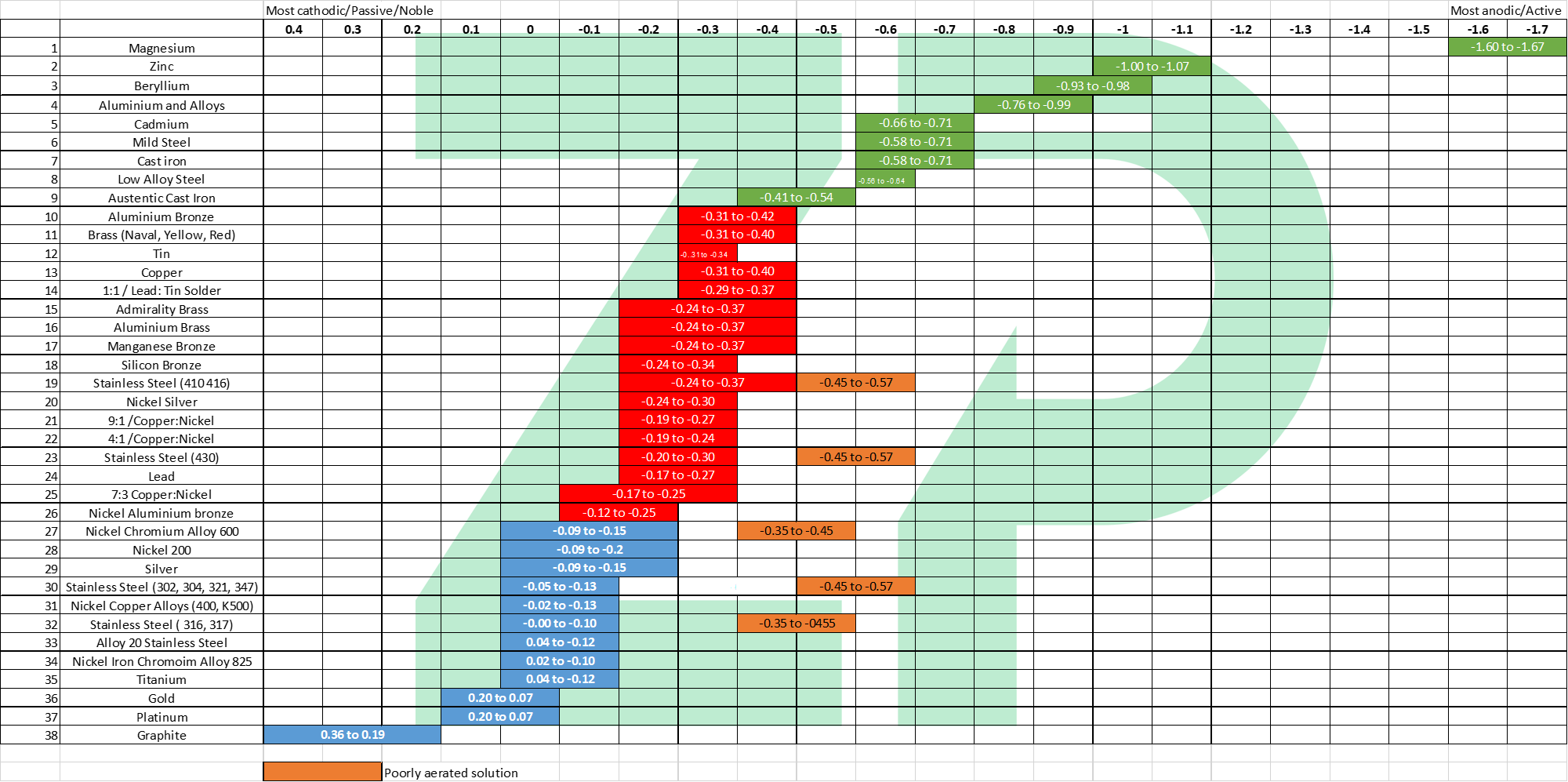
Galvanic Corrosion For Medical Devices Zimmerandpeacock
Medical Device Shelf life.

. Q10 value can be changed however default is Q1020 we recommend holding this. It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. Sterile packaged medical devices are usually date labeled or have a pull date and may have a shelf life as defined by the 1991 FDA.
Real Time Aging oftentimes referred to as Shelf-Life Testing is exactly what the name implies. These accelerated tests help pinpoint possible seal and burst. Accelerated-aging tests are employed to generate this data for.
This testing can identify film delineation and leaks. Materials and packaging impacts the shelf life of the device. Every medical device is required to be labeled with an expiration date that is supported by shelf-life data.
Accelerated Aging is a process of putting packaged products into a chamber elevating the test temperature to claim a specific expiration date for a medical device product or package. Donohue J and Apostolou S Shelf-Life Prediction for Radiation-Sterilized Plastic Devices Med Dev Diag. Westpak maintains dedicated space.
Real-Time Aging for Medical Devices. In order to calculate expiry date you should look at Production Date on your wrapping and write it. A written procedure for establishing and monitoring shelf life of medical devices should include the following.
However sometimes it may take. Accelerated aging parameters with results supported. Therefore the FDA requires medical device manufacturers to determine a products shelf life before sending the product to market in the United States.
Type the target shelf life Days 2. This online service helps you to know how long your product is in good condition. Shelf Life of Sterile Medical Devices.
Shelf-life is the length of time you can expect a product to look and act as expected and to stay safe for use. The shelf life of medical devices is determined by putting a device through a variety of testing procedures and many engineers follow this step by procedure to avoid any. The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life.
Download the Final Guidance Document. A plan for the storage of shelf life samples including storage conditions. Shelf Life of Medical Devices April 1991.
Return to learning center. This length of time varies depending on the type of. This testing is conducted at ambient conditions in actual real time in order to analyze the conditions of packages andor products and the effects that time has on them.
Determining a medical devices shelf life the term or period during which it remains. Organizational units responsible for the various phases of. Accelerated aging testing is an FDA requirement for medical biomedical and pharmaceutical products.
Type desired TAA TRT Values. Clark GS Shelf Life of Medical Devices FDA DSMA report April 1991.

Predicting Shelf Life From Accelerated Aging Data The D A And Variabl Mddionline Com

High Temperature Aging Tests Choosing A Temperature Packaging Compliance Labs

Life Science Outsourcing Inc Need Help With Your Accelerated Aging Data Our Calculator Generates A Table Of Values For Calculations Based Upon Astm F1980 Accelerated Aging Of Sterile Medical Device Packages

Calculate Expiry Date In Excel Product Expiry Formula In Excel

Accelerated Aging Shelf Life Testing Element
Challenges And Opportunities In Software Driven Medical Devices Nature Biomedical Engineering

How To Determine The Shelf Life Of Medical Devices Previous Magazine
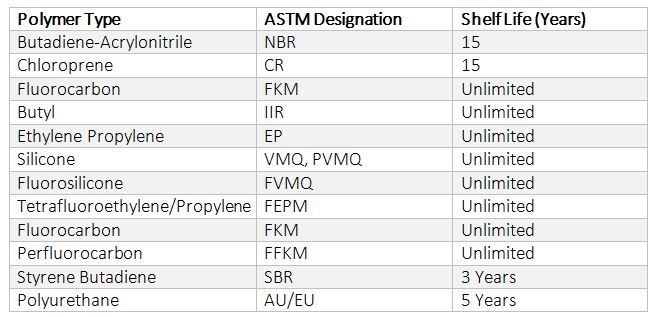
How To Calculate Shelf Life Of Elastomers Hot Topics

Sharp Calculators Shrel1901 El 1901 12 Digit Paperless Printing Calculator 1 Each White Walmart Com

Accelerated Aging Shelf Life Testing Element

Calculation Of Shelf Life Of Packaging Material With Ficks First Law 1855
Accelerated Aging Calculator Shelf Life Calculator Packaging Compliance Labs

Why Do We Need To Batch Produce And Use Batch Numbers

Amazon Com 20 Count Cr2032 Lithium Coin Cell Battery 3v Blister Packed Cr2032 Button Battery For Small Devices Long Lasting Power 8 Year Storage Shelf Life Child Secure Packaging Pack Of 20 Health

Calculation Of Expiry Date Shelf Life By Accelerated Stability Study Method In Hindi Youtube

Medical Package Testing Lab Iso 17025 Certified Packaging Compliance Labs
Long Term Stability Predictions Of Therapeutic Monoclonal Antibodies In Solution Using Arrhenius Based Kinetics Scientific Reports